The products meant to keep you clean may actually be putting your health in danger. A shocking bacterial contamination has forced the recall of over a dozen well-known soap and sanitizer products after connection to life-threatening sepsis was discovered. From lotions to foam soaps, these everyday essentials may pose serious risks, especially for those with compromised immune systems. Read on to uncover which products are affected, what symptoms to watch out for, and how you can protect yourself and your loved ones.
DermaRite announces urgent nationwide recall of hygiene products
On August 8, 2025, DermaRite Industries LLC issued a critical health alert, announcing a voluntary recall of multiple soap and hand sanitizer products after dangerous bacterial contamination was detected. The urgent recall impacts more than a dozen items sold across the United States and Puerto Rico, following tests that confirmed the presence of Burkholderia cepacia.
This bacterium poses a serious health risk, as it is highly resistant to antibiotics and capable of causing severe, potentially life-threatening infections. Commonly found in soil and water, the germs can also spread easily from person to person.
In a company statement, DermaRite confirmed that products such as DermaKleen lotion soaps, PeriGiene antiseptic, and KleenFoam soap are included in the recall. Health officials are urging consumers to review their supplies, as infections may cause symptoms including fever and fatigue.
How to check if your soap is part of the recall
To determine whether a DermaRite product you own is included in the recall, check the brand name and lot number printed on the label or packaging. The company has published a detailed list of affected items, which includes reorder numbers, lot numbers, and expiration dates. These details can be found in the images provided by the U.S. Food and Drug Administration (FDA) below.
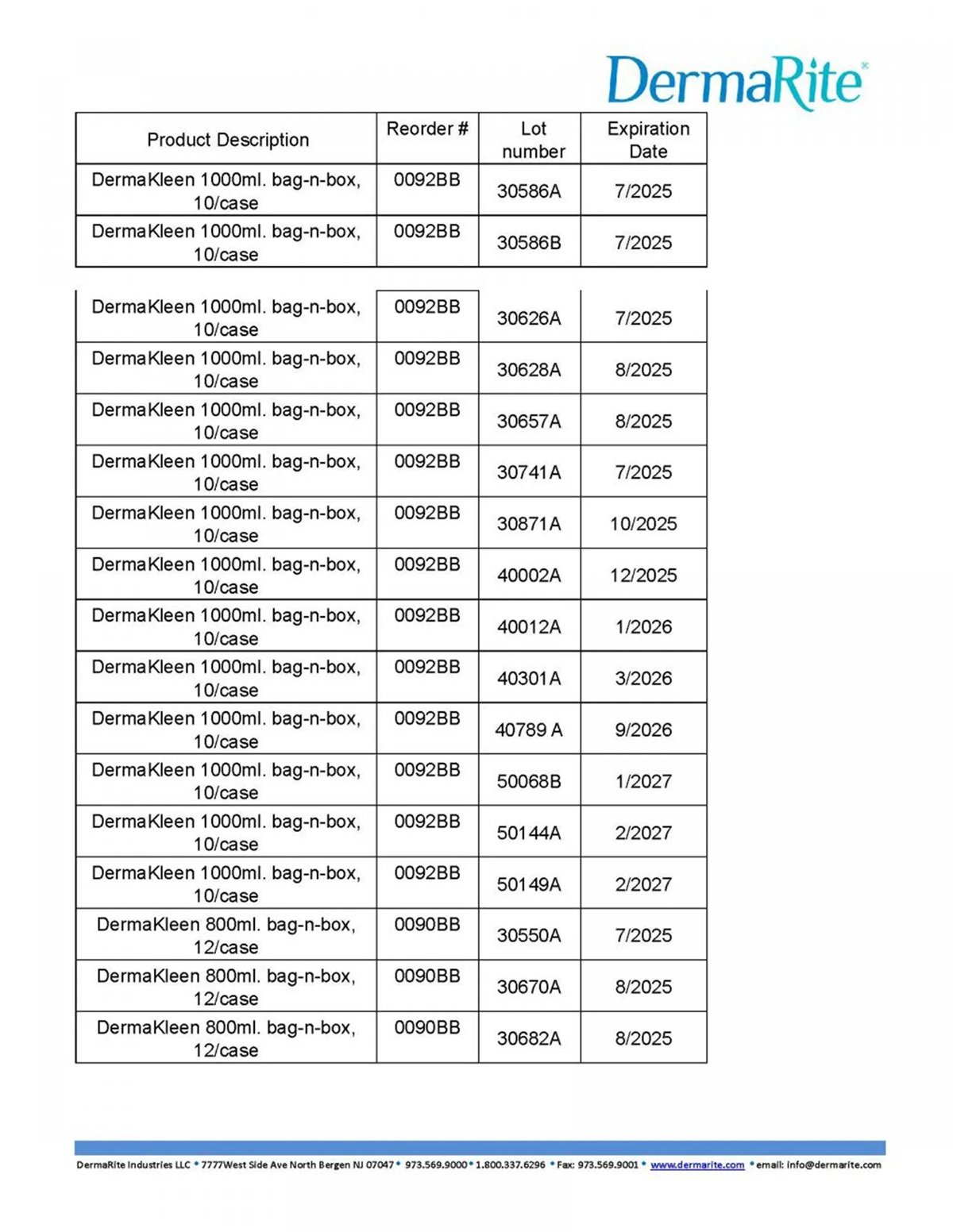
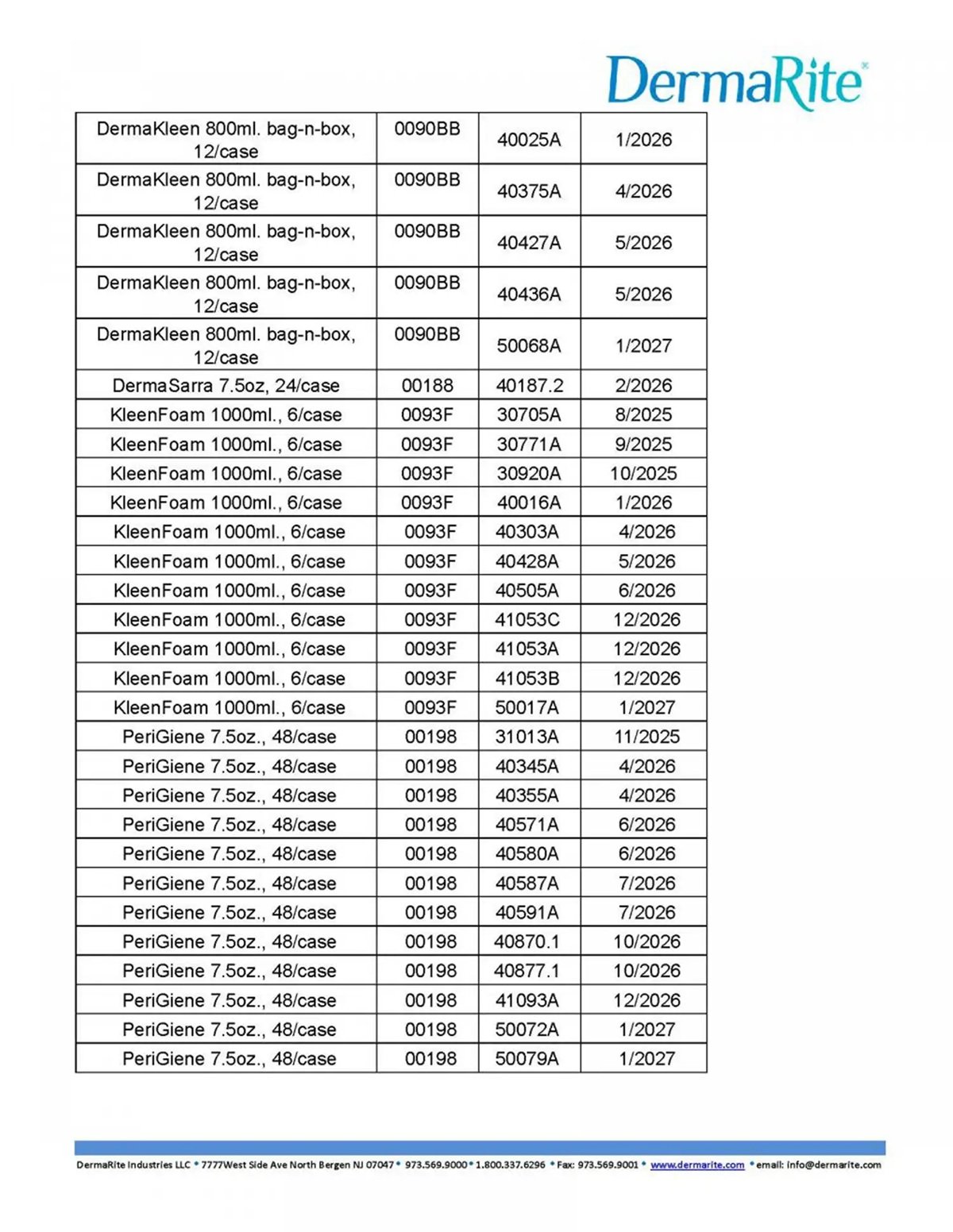
If you believe you’ve experienced any health issues after using the recalled products, seek medical attention right away. Contact your physician or healthcare provider to discuss any potential side effects.
The FDA also urges consumers to report any adverse reactions through its MedWatch Adverse Event Reporting program, available online.
For general questions regarding the recall, DermaRite advises reaching out directly via email at voluntary.action@dermarite.com
Health risks linked to the recalled products
DermaRite has warned that the recalled soaps may pose health risks for all users, with those who have weakened immune systems at the greatest risk. In healthy individuals with small cuts or skin abrasions, exposure to the bacteria may lead to localized infections. However, in people who are immunocompromised, the threat is far more serious, as the bacteria can enter the bloodstream and potentially trigger sepsis, a life-threatening medical emergency.
Sepsis develops when the body’s immune system overreacts to an infection, releasing chemicals into the blood that can cause widespread inflammation, tissue damage, organ failure, and even death. Symptoms may include fever, rapid heartbeat, shortness of breath, or confusion, and without immediate medical care, the condition can escalate quickly. According to the Centers for Disease Control and Prevention (CDC), at least 1.7 million adults in the U.S. develop sepsis each year, and roughly 350,000 die in hospitals or are discharged to hospice care as a result.
As of August 8,2025, DermaRite reported no confirmed adverse reactions connected to the recalled products. Even so, the company has stressed the urgency of discontinuing use and has already notified distributors and customers about the recall.

